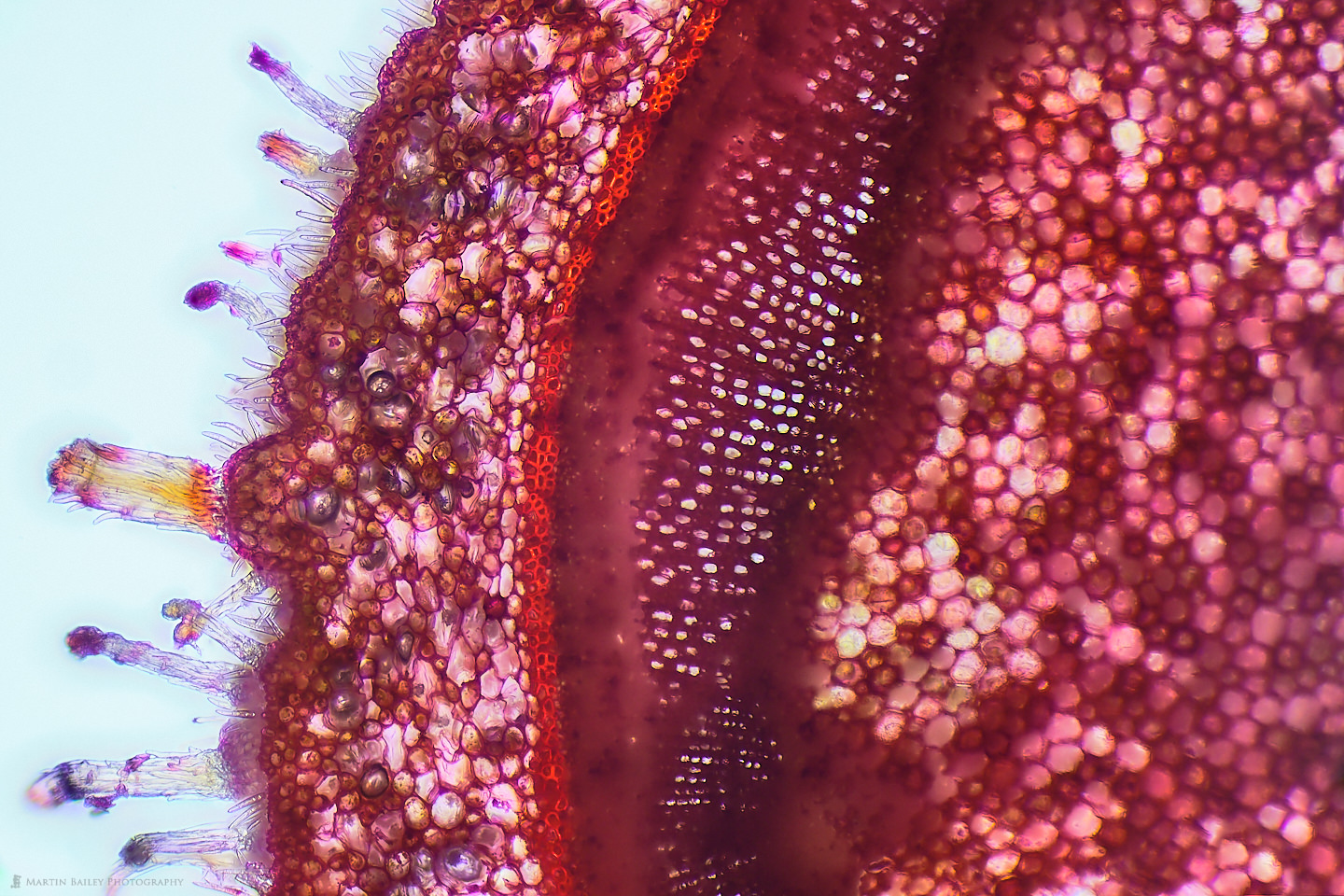
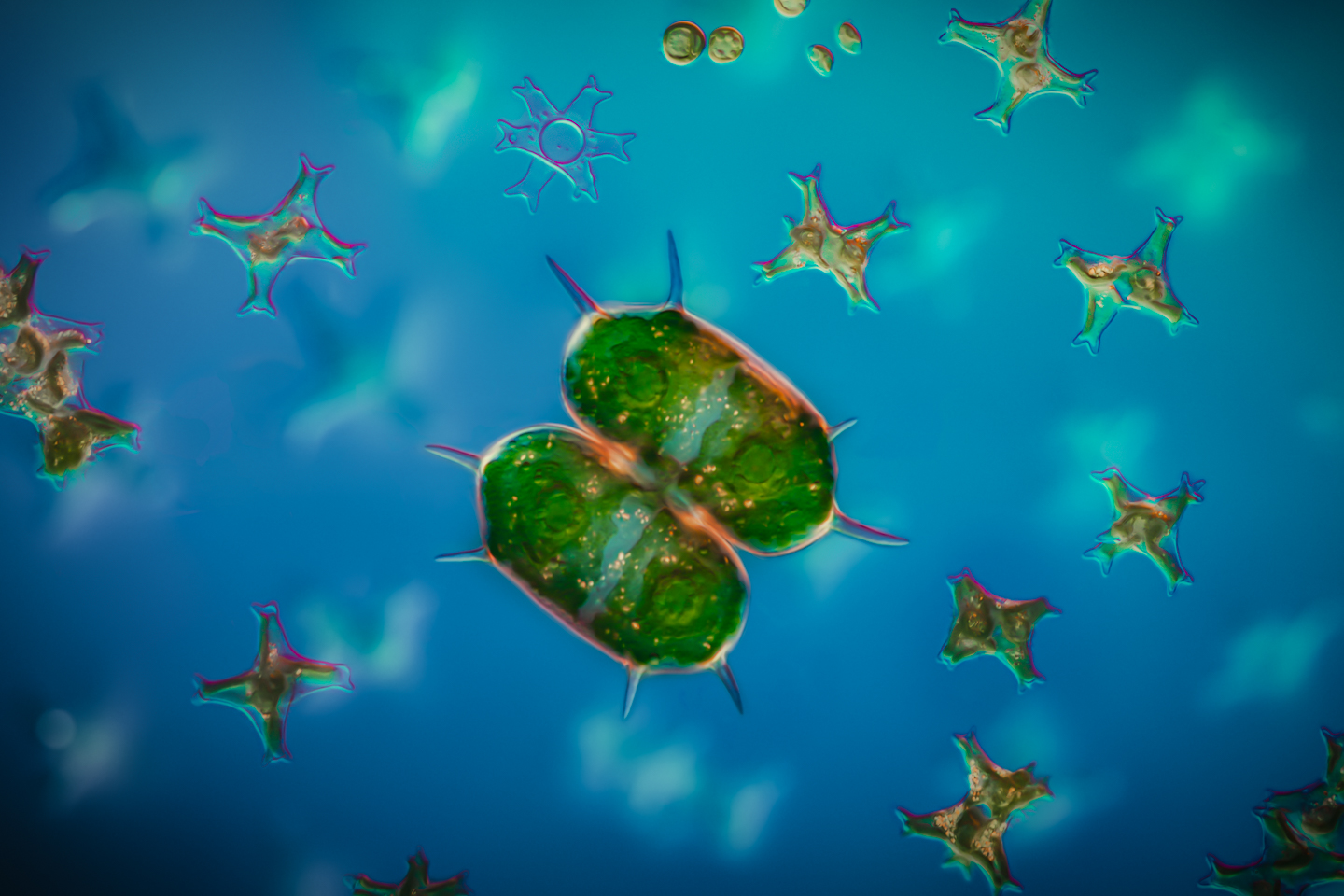
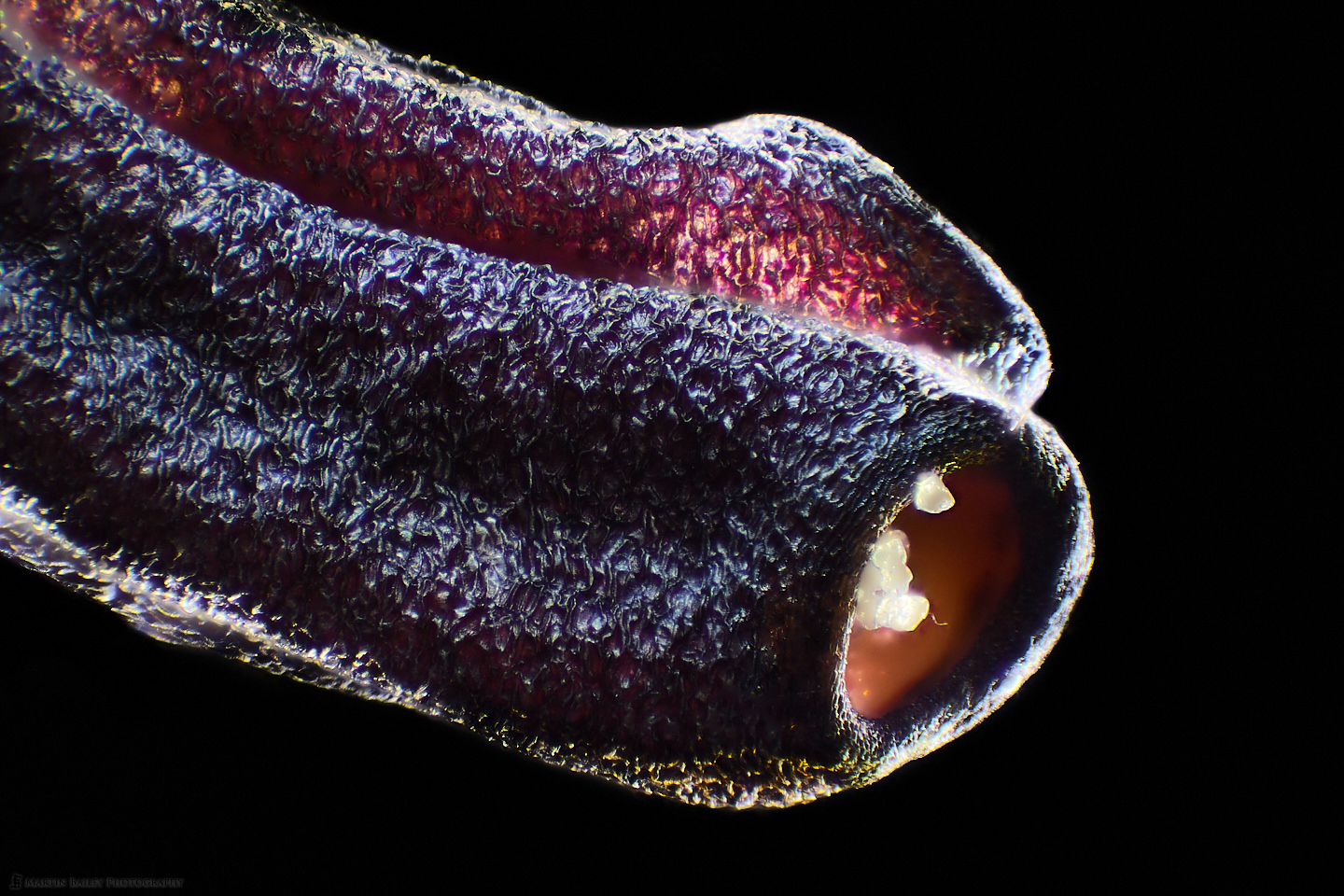
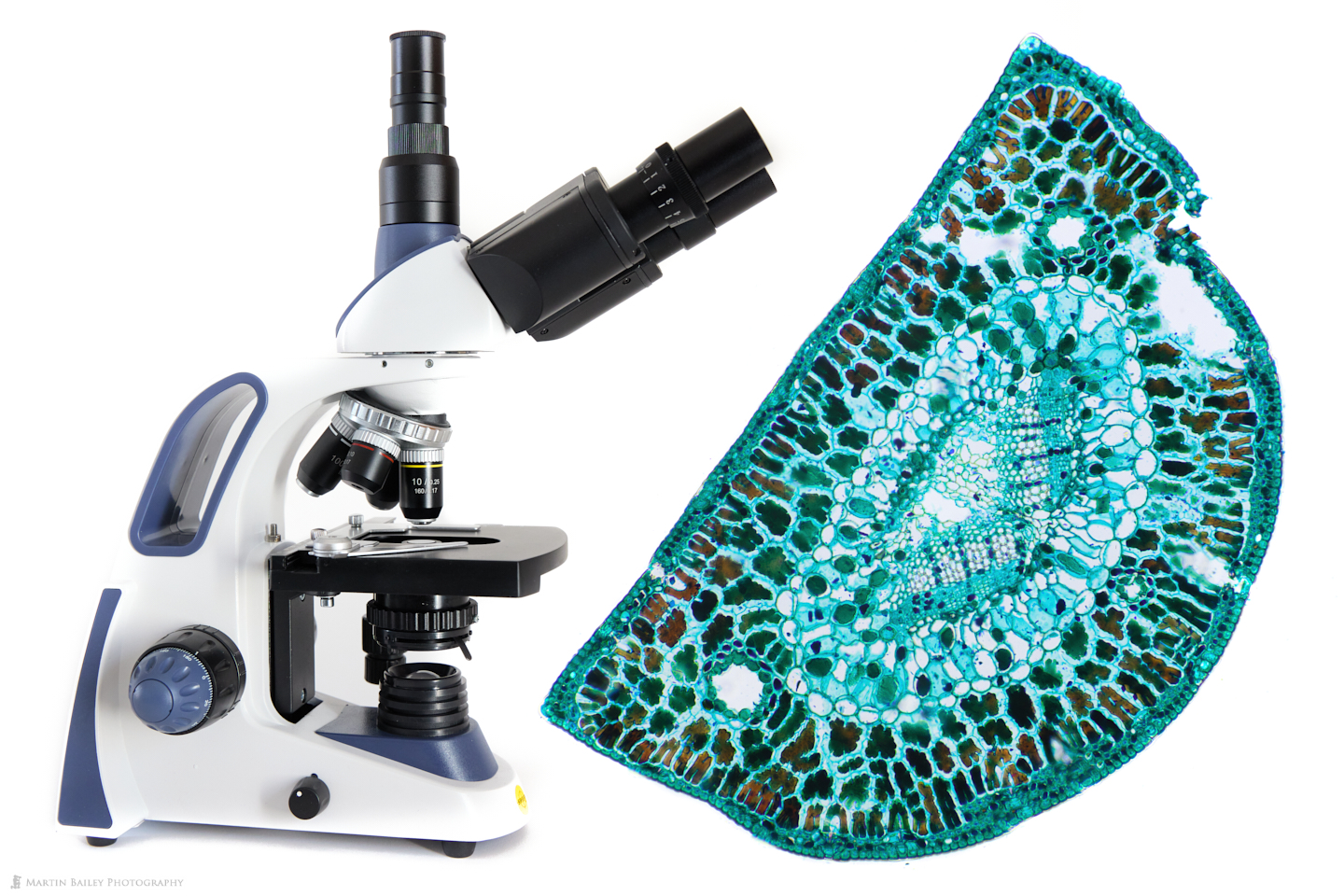
In this week’s post, I share how I’ve been using a microtome and various stains to prepare specimen sections to photograph.
microphotography
In Conversation with Award-Winning Microphotographer Håkan Kvarnström (Podcast 750)
I’m honored to be able to bring you a conversation with one of the top microscope photographers in the field, the award-winning Håkan Kvarnström.
Preparing and Photographing a Housefly with a Microscope (Podcast 748)
Today I walk you through the process of preparing a housefly to be photographed, and some tips on focus-stacking insects using a microscope.
The Moral Dilemma of Killing Insects for Photographs (Podcast 747)
Today we discuss killing insects to photograph them. I recently killed a housefly and photographed it. They usually just go into the trash.
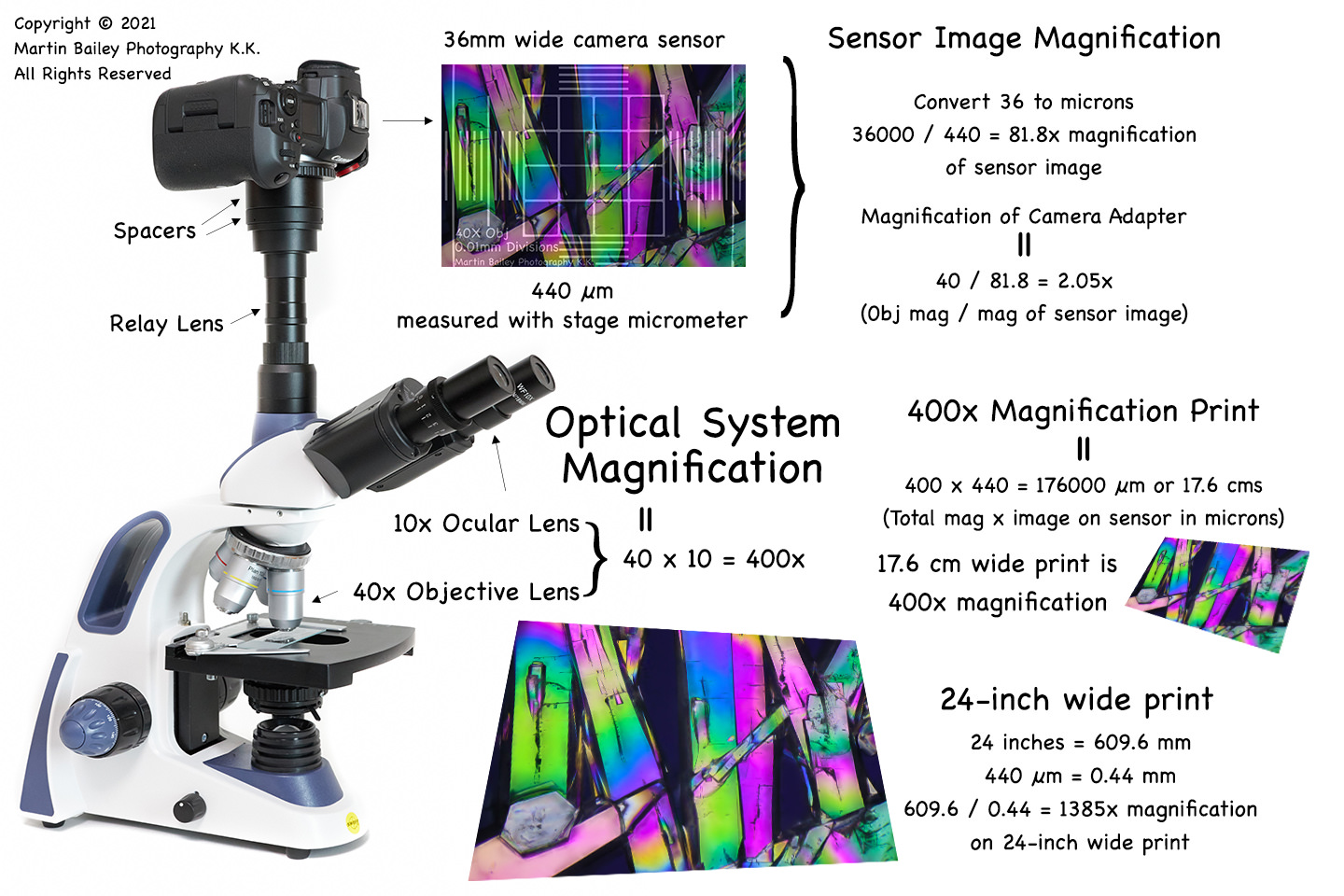
Microscope Calibration, Micrometry and Magnification (Podcast 746)
The curious geek in me just figured out how to calculate the size of my microscopic specimens and magnification of my camera adapter and print sizes.
Using Helicon Focus for Microphotography (Podcast 742)
In this post, I share how I’m using Helicon Remote and Helicon Focus to create focus-stacked images in my recent adventure into microphotography.
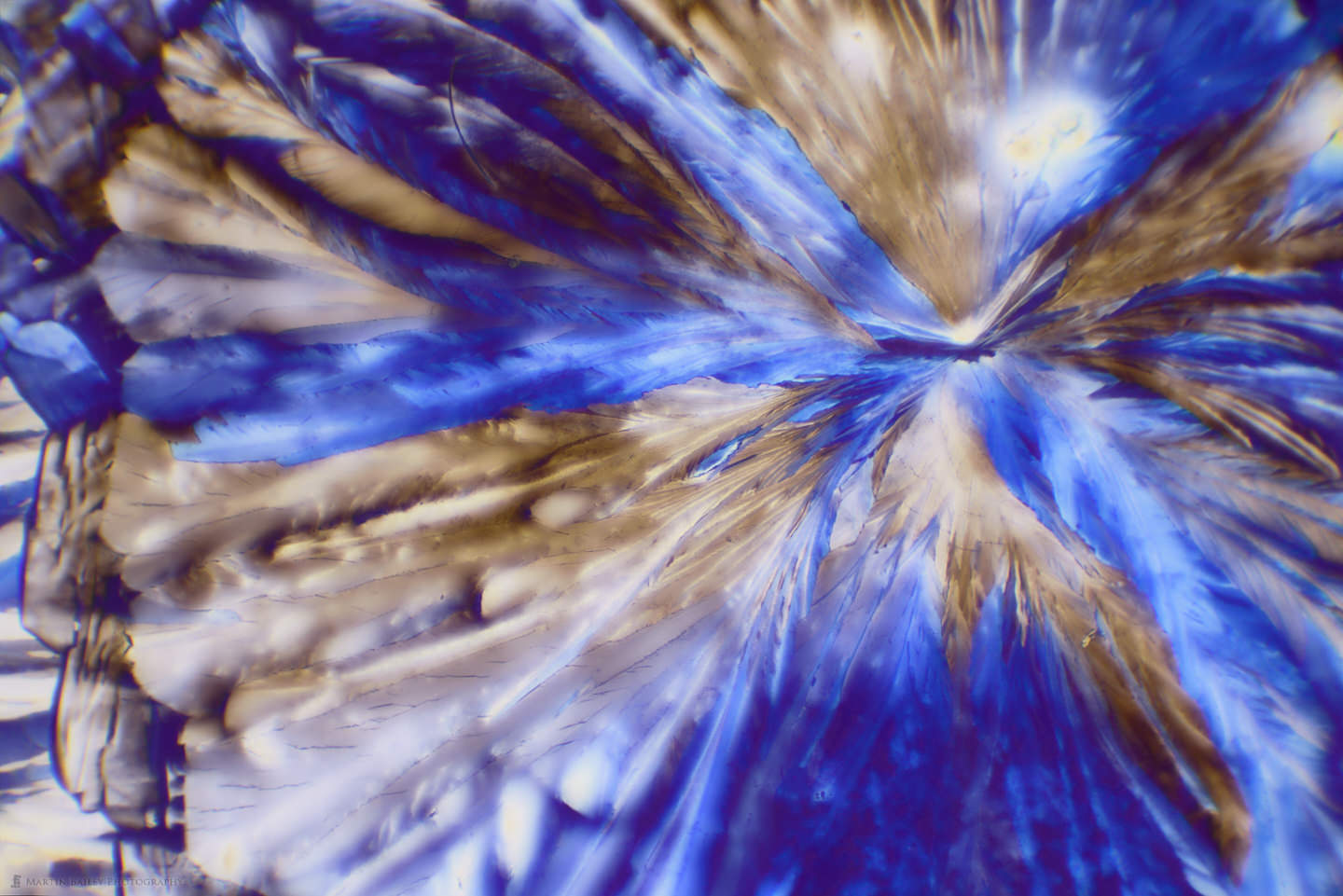
Creating Micrographs of Polarized Citric Acid Crystals (Podcast 740)
In this post, I share the process of making citric acid crystals, as well as a number of micrographs of the crystals at 40X and 100X magnification.
Latest Rabbit Hole Update – Micrography (Podcast 738)
I’ve dived head-first down the rabbit hole of micrography and today share where I’m at, my first attempts, and plans for my near future exploration.